About BIOVANCE®
WE’RE MAKING OUR MARK BY BARELY LEAVING ONE
BIOVANCE is decellularized, dehydrated human amniotic membrane (DDHAM) derived from the placenta of a healthy, full-term pregnancy.
BIOVANCE Is Unique
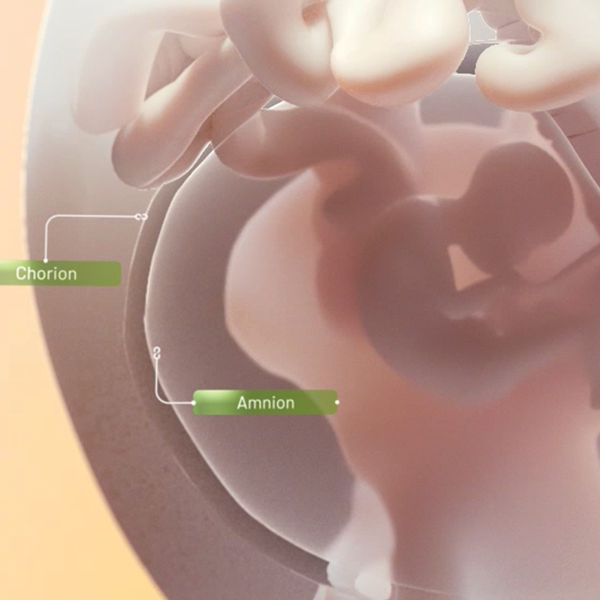
BIOVANCE is unique because it is prepared from the amnion—the part of the amniotic sac closest to the developing embryo. The amnion is excised from the chorion layer of the placenta, washed, and then sterilized so that it contains only what’s needed.
The natural properties of the amniotic membrane support the growth and development of the baby. For 9 months, the amniotic membrane:
- Forms the innermost lining of the placenta during gestation1
- Is the tissue closest to the baby throughout development
- Serves as a barrier to minimize risk of injury to the baby1-3
BIOVANCE® is a decellularized, dehydrated human amniotic membrane (DDHAM) with a preserved natural epithelial basement membrane and an intact extracellular matrix structure. The epithelial basement membrane and extracellular matrix of this allograft provide a natural scaffold. BIOVANCE provides a protective cover from the surrounding environment.
BIOVANCE components
- Collagen I, II, III, IV
- Elastin
- Glycosaminoglycans
- Fibronectin
- Laminin
- Proteoglycans
See BIOVANCE in Action
Watch a brief video animation that illustrates the mechanism of action of BIOVANCE.

Easy to Use
BIOVANCE is easy to use, making it ideal for surgical and nonsurgical settings.
Easy to apply
- No preparation needed – No thawing, rinsing, or soaking required prior to use
- Flexible form – Conforms easily to irregular surfaces; can be wrapped around tendons or placed between adjacent tissues
- Bidirectional orientation – Prevents the need for specific placement of BIOVANCE on the wound; can be applied with either side facing the wound
- Adheres without sutures – Can be fastened by all surgical means if physician chooses to do so
Convenient storage
- Ambient room-temperature storage in a clean, dry environment – No refrigeration necessary
- 10-year shelf life – Eliminates the need for preordering
Immunologically Inert Tissue
BIOVANCE is immunologically inert tissue that has been minimally processed.
- BIOVANCE contains no antigens7
- Tissue derived from the amniotic membrane is cleaned and preserved without altering its native matrix architecture
Additional safety features
Tissue used in processing BIOVANCE:
- Has been procured, processed, and tested in accordance with standards established by the American Association of Blood Banks (AABB) and the United States Food and Drug Administration (FDA)
- Passed safety testing for cytotoxicity, hemolysis, irritation, endotoxins, and pyrogenicity
- Utilizes a barcode tracking system for optimal safety monitoring and to enhance patient and practitioner confidence
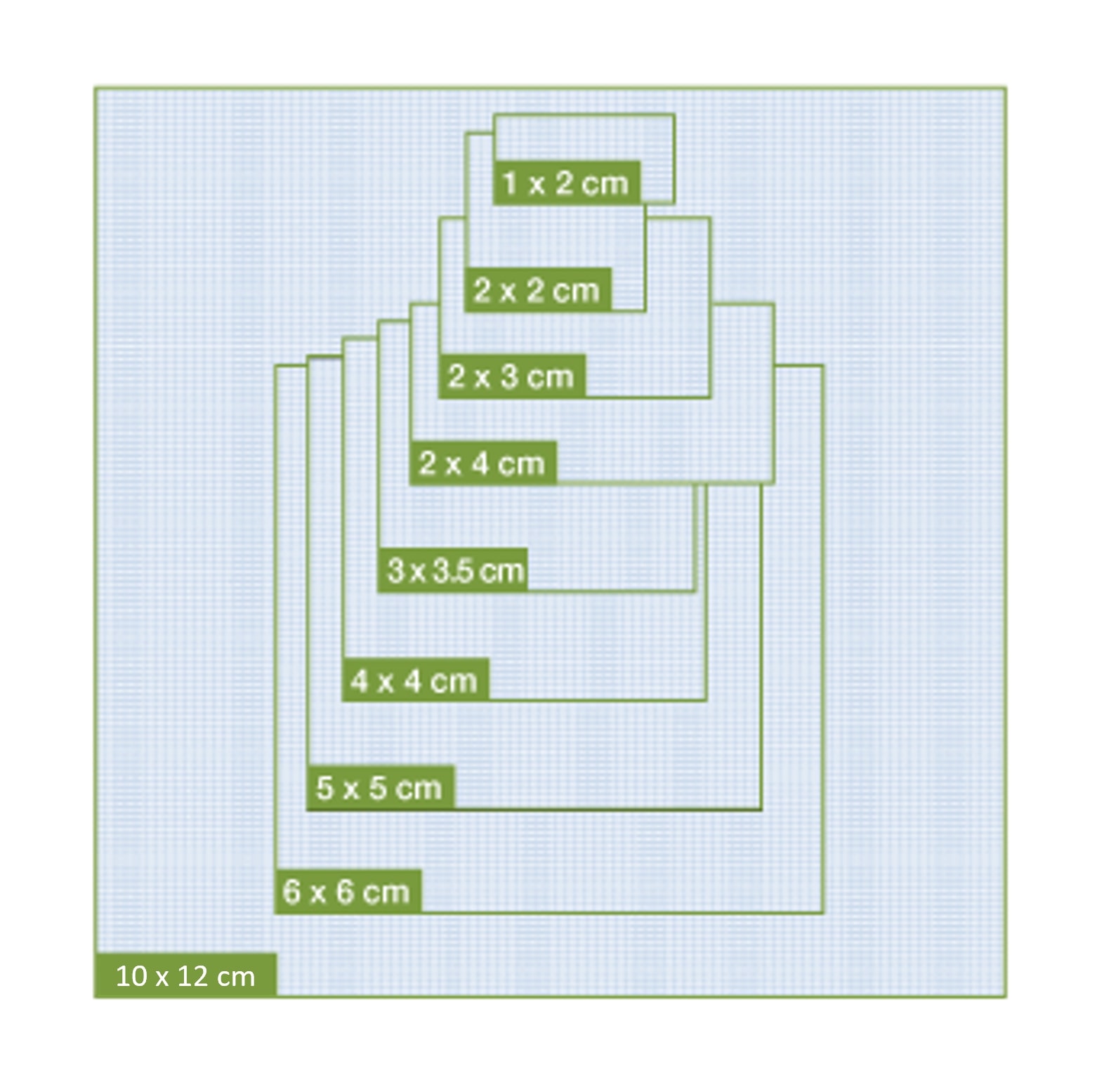
Available in 8 different sizes for application flexibility
1×2 cm
Product Code DHAM0012
2×2 cm
Product Code DHAM0022
2×3 cm
Product Code DHAM0023
2×4 cm
Product Code DHAM0024
3×3.5 cm
Product Code DHAM0035
4×4 cm
Product Code DHAM0044
5×5 cm
Product Code DHAM0055
6×6 cm
Product Code DHAM0066
10×12 cm
Product Code DHAM1012
Clinical Outcomes Gallery
LEGAL DISCLAIMER By submitting your information and acknowledging your agreement, you agree to the terms and conditions outlined Access Agreement below.
ACCESS AGREEMENT Thank you for your interest in Celularity, Inc. (“Celularity”). This Access Agreement (the “Agreement”) establishes the terms and conditions by which Celularity is willing to grant you (“Visitor”) access to specific information regarding Celularity’s products. By clicking “I AGREE,” you acknowledge that you have read and accept the terms and conditions of this Agreement in its entirety.
A. Grant of License to Access. In consideration of the acceptance of the terms, conditions, and limitations provided in this Agreement, Celularity grants to you (“Visitor”) a nontransferable, nonexclusive license to use the information contained therein (the “Clinical Outcomes”) solely for Visitor’s own internal use in conducting evaluation of Celularity’s products for professional clinical use consistent with the approved product labelling. This license is effective for (and limited to) the duration of your viewing of the Clinical Outcomes on Celularity’s website.
B. Permitted Access and Use. Visitor acknowledges and agrees that it is permitted to access the Clinical Outcomes solely and Visitor agrees not to make any copies (including screen shots or other forms of data capture) of any information contained therein, except in connection with viewing the Clinical Outcomes through Celularity’s website. For the avoidance of doubt, Visitor may not modify, adapt, reverse engineer, or create derivative works of the Clinical Outcomes.
C. Restrictions and Limitations. Visitor represent and warrant that you: (a) agree to use the Clinical Outcomes solely for the purposes set forth herein; (b) agree not to use the for for any other purpose, including competitive marketing, the creation of derivative works, or data-mining; (c) will take all reasonable steps to protect the Clinical Outcomes from theft or use contrary to the terms of this Agreement; and (d) agree not to reproduce, deactivate or bypass securities systems on the Celularity website. Any requests for rights of use the Clinical Outcomes other purposes should be directed to Celularity at MedicalAffairs@Celularity.com.
D. Reservation of Rights. Title, ownership and all other rights in patents, copyrights, and trade secrets in the Clinical Outcomes shall be owned exclusively by Celularity. Celularity reserves all rights not specifically transferred to Visitor by this Agreement. Celularity’s ownership rights shall extend to the Clinical Outcomes, all copies thereof, and any Information regardless of whether Visitor owns the media on which the Clinical Outcomes, any copies thereof, is recorded and regardless of whether such copies of the Clinical Outcomes may be authorized or unauthorized.
E. Disclaimer of Warranties. No warranties of any kind, whether express or implied, are given by Celularity with respect to the website or the Clinical Outcomes or any use thereof, and the information is provided on an “AS IS” basis and for educational purposes only. With respect to the products, Celularity does makes no representations or warranties as to the performance or suitability of any product for any general or specific purpose, indication, treatment or condition. CELULARITY HEREBY EXPRESSLY DISCLAIMS ALL SUCH WARRANTIES, INCLUDING ANY IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE AND ANY WARRANTIES ARISING OUT OF COURSE OF PERFORMANCE, COURSE OF DEALING OR USAGE OF TRADE. In no event will Celularity be liable to Visitor or any third party for any loss of profits, business interruption, or loss of business information; any incidental, special, exemplary, or consequential damages; or any claims or demands brought against Visitor, even if Celularity has been advised of the possibility of such damages.
F. Visitor Data. Visitor agrees that Celularity will have the right to generate data from Visitor regarding Visitor’s access and use of the Clinical Outcomes and may use such data for any business purpose (including product marketing) during or after the term of this Agreement.
G. General Terms and Conditions. All matters relating to the website and this Agreement and any dispute or claim arising therefrom or related thereto (in each case, including non-contractual disputes or claims), shall be governed by and construed in accordance with the laws of the State of New Jersey without giving effect to any choice or conflict of law provision or rule (whether of the State of New Jersey or any other jurisdiction).
IF YOU ARE ENTERING INTO THIS AGREEMENT WITHIN THE SCOPE OF YOUR EMPLOYMENT OR IN CONNECTION WITH YOUR ENGAGEMENT AS AN INDEPENDENT CONTRACTOR, THEN THE TERM “VISITOR” INCLUDES YOUR EMPLOYER OR PRINCIPAL CONTRACTOR, AS APPLICABLE, AND YOU WARRANT AND REPRESENT TO CELULARITY, INC. THAT YOU ARE AUTHORIZED TO ACCEPT THIS AGREEMENT ON SUCH EMPLOYER’S OR PRINCIPAL CONTRACTOR’S BEHALF.
Connect With Celularity
For product questions or ordering information, please contact us at customerservice@celularity.com or call us at 1-844-963-2273.
For medical inquiries, please contact us at medicalaffairs@celularity.com
References:
- Fetterolf DE, Synder RJ. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds. 2012;24(10):299-307.
- Bhatia M, Pereira M, Rana H, et al. Mechanism of cell interaction and response on decellularized human amniotic membrane: implications in wound healing. Wounds. 2007;19(8):207-217.
- Arechavaleta-Velasco F, Marciano D, Díaz-Cueto L, Parry S. Matrix metalloproteinase-8 is expressed in human chorion during labor. Am J Obstet Gynecol. 2004;190(3):843-850.
- Faulk WP, Matthews R, Stevens PJ, et al. Human amnion as an adjunct in wound healing. Lancet. 1980;1(8179):1156-1158.
- Ganatra MA. Amniotic membrane in surgery. J Pak Med A. 2003;53(1):29-32.
- Portmann-Lanz CB, Ochsenbein-Kölble N, Marquardt K, et al. Manufacture of a cell-free amnion matrix scaffold that supports amnion cell outgrowth in vitro. Placenta. 2007;28(1):6-13.
- Niknejad H, Peirovl H, Jorjani M, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88-99.

BIOVANCE® and Celularity® are registered trademarks of Celularity Inc.
© 2021 Celularity Inc.