
Within 24 hours, cells readily attached to BIOVANCE, but not dHACM1
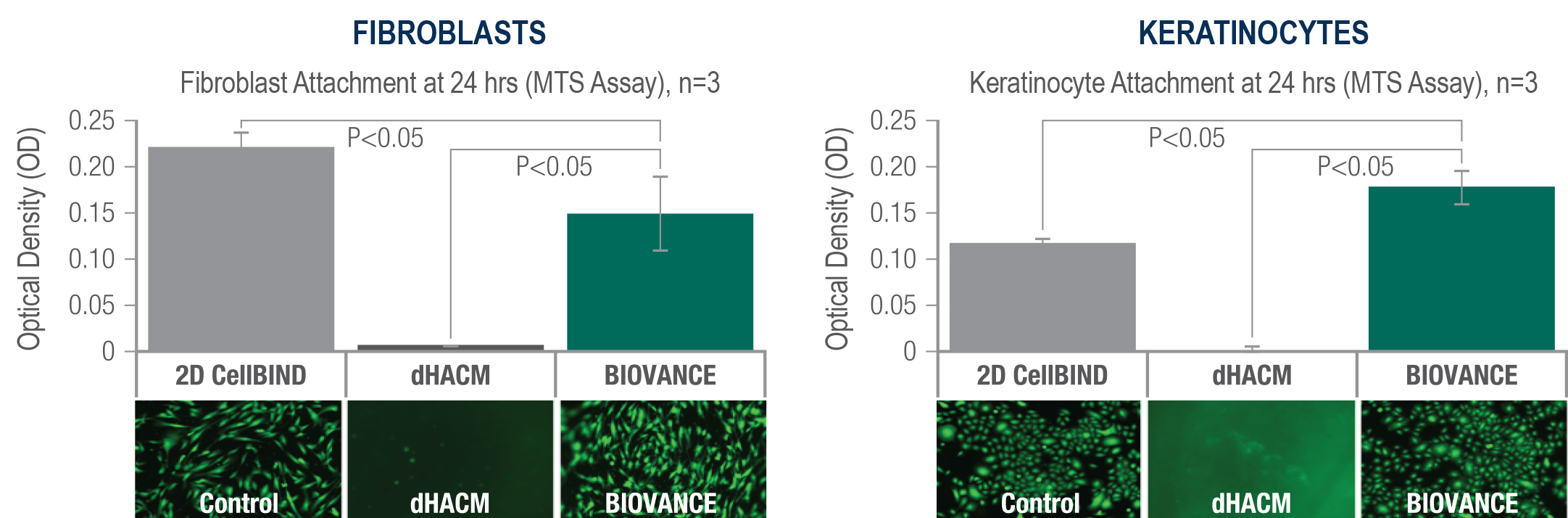
In in vitro data, BIOVANCE served as an inert scaffold with an intact basement membrane that supported a high level of fibroblast and keratinocyte attachment vs dHACM, which had no attachment of either cell type
Fibroblasts that attach to and grow on BIOVANCE release growth factors in vitro that support wound closure1
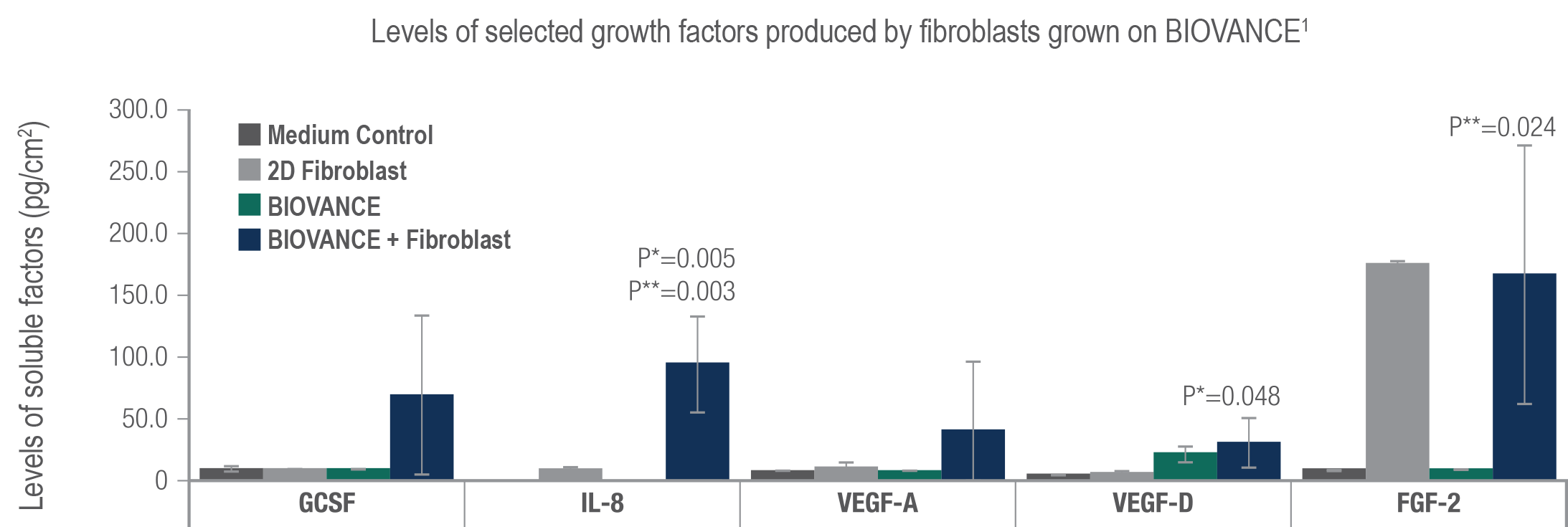
- Growth factors, among other key molecules released by attached fibroblasts, may support key events in wound healing such as cell survival, wound closure, and angiogenic blood vessel formation
- Once growth factors were released, measured cell metabolic activity showed the revival of senescent endothelial cells and keratinocytes
REFERENCE: 1. Bhatia M, Pereira M, Rana H, Stout B, Lewis C, Abramson S. The mechanism of cell interaction and response on decellularized human amniotic membrane: Implications in wound healing. Wounds. 2007;19(8):207-217.

